An alkene formula with 20 carbon atoms. Carbon atoms can bond to other carbon atoms.

Carbon And Carbon Bonding Biology For Non Majors I
Carbon atoms can only form triple bonds with other carbon atoms.

. Carbon atoms only have three electrons available for bonding. Why Are The Number Of Carbon Atoms Used As Prefixes Quora. C C-1 is a quaternary carbon and C-3 is a secondary carbon.
In a solution the amino. Carbon atoms can bond to other carbon atoms. In a parallel circuit the.
A The methyl group at C-3 is equatorial. Sp sp and sp2 respectively Question 2 Which of the following accurately represents the molecular geometry for the carbon atoms in C 2 H 6 ethane C 2 H 4 ethene and C 2 H 2 ethyne. The outer shell is incomplete with four electrons.
B C-1 is a tertiary carbon and C-3 is a primary carbon. When carbon atoms bond to other carbon atoms they can only form triple bonds. Click hereto get an answer to your question Select the correct statements about cubic structure of diamond1.
Carbon atoms only have three electrons available for bonding. Carbon atoms only have three electrons available for bonding. A hydrocarbon chain with a carboxyl group at both ends and no double bonds between the carbon atoms.
Which of the following accurately describes carbon atoms. If molecules of ethanoic acid contain 2 carbon atoms 4 hydrogen atoms and 2 oxygen atoms the molecular formula for this compound could be written as C 2 H 4 O 2. It contains both an amino functional group and a carboxyl functional group.
Sp sp3 and sp2 respectively B. Carbon atoms can bond to other carbon atoms. Carbon atoms can only form triple bonds with other carbon atoms.
Approximate packing fraction of unit cell is 3 pi16. Atomic radius of carbon is 3 a8 where a is edge length of cube4. Which of the following accurately describes carbon atoms.
1 a six-membered ring with 3 double and 3 single bonds 2 a ring described as 135-hexatriene 3 identical bonds between all 6 carbon atoms with 6 electrons moving freely 4 an extremely reactive six-membered ring 5 a six-membered ring with easily. Carbon atoms only have three electrons available for bonding. Carbon atoms are only found in two of the four main macromolecules.
When carbon atoms bond to other carbon atoms they can only form triple bonds. Which phrase most accurately describes the structure common to all aromatic compounds. Carbon atoms can bond to other carbon atoms.
Which of the following accurately describes carbon atoms. No comments for 15 Which of the Following Accurately Describes Carbon Atoms Post a Comment. D C-1 is a tertiary carbon and C-3 is a secondary carbon.
A a six-membered ring with 3 double and 3 single bonds B a ring described as 135-hexatriene C identical bonds between all 6 carbon atoms with 6 electrons moving freely D a six-membered ring with easily broken carbon-carbon bonds E none of the above Answer. Without carbon life will be non-existent. In a parallel circuit theres only one path for the current to travel.
Single double and triple bonds 4. Carbon atoms can bond to other carbon atoms. In order to obtain a stable outer shell of electrons carbon forms four.
Carbon atoms are only found in two of the four main macromolecules. Which of the following accurately describes carbon atoms. Carbon atoms can only form triple bonds with other carbon atoms.
Carbon atoms are only found in two of the four main macromolecules. Sp2 sp and sp3 respectively D. Carbon atoms are only found in two of the four main macromolecules.
Sp3 sp2 and sp respectively C. Yes the breakdown of food describes the process of cell respiration. When carbon atoms bond to other carbon atoms they can only form triple bonds.
Carbon atoms only have three electrons available for bonding. Carbon atoms are only found in two of the four main macromolecules. It provides the framework for all living organisms on Earth josh176 josh176 05312017 Biology High School Which is the following accurately describes carbon atoms.
Each carbon is surrounded by four more carbon atoms3. The inner shell is complete with two electrons. Carbon atoms are only found in two of the four main macromolecules.
A hydrocarbon chain with a carboxyl group at both ends and one or more double bonds between the carbon atoms D. The answer is B. Zinc is the limiting reagent.
1 See answer josh176 is waiting for your help. Which of the following accurately describes carbon atoms. Zinc is the limiting reagent.
Which of the following statements is a correct description of the most stable conformation of 113-trimethylcyclohexane. Which of the following terms accurately describes the energy associated with the process. Lig Li g e-a electron affinity b binding energy c ionization energy d electronegativity e none of these 5.
Carbon Atom An Overview Sciencedirect Topics Share. Add your answer and earn points. In a series circuit the current can flow through only one path from start to finish.
Carbon atoms can form chains and branches with each other and other atoms can attach to these. 3 hydrocarbon chains joined to a glycerol molecule and one or more double bonds between the carbon atoms E. Popular Total Pageviews Powered by Blogger Labels Accurately an Aneka Baju Berapa Brand Buat Bulan Cafe.
Co is used as an energy source in photosynthesis C. The species that contains 24 protons 26 neutrons and 22 electrons would be represented by the symbol. Which of the following statements accurately describes the situation.
Effective number of atoms present in a diamond cubic cell is 8 2. In a series circuit the amount of current passing through each part of the circuit may vary. Which of the following accurately describes circuits.
Is carbon dioxide a waste product formed in the breakdown of food. Carbon atoms can bond to other carbon atoms. A 50 V 3 b 26 Cr 2 c.
The products of respiration are carbon dioxide and water.

Carbon Atom An Overview Sciencedirect Topics

Carbon Atom An Overview Sciencedirect Topics
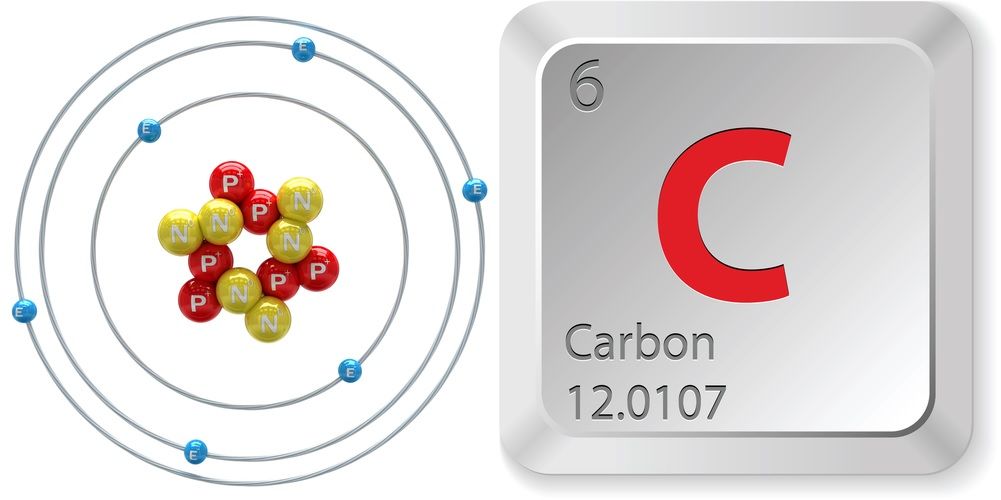
Carbon Facts About An Element That Is A Key Ingredient For Life On Earth Live Science
0 Comments